CSL
CSL1 was a randomized clinical trial where, in the period 1962-69, 488
patients with liver cirrhosis were treated with either the active drug
prednisone (251 patients) or placebo (237 patients). The purpose of
the trial was to evaluate the effect of treetment on survival. After
randomization patients were followed to either death, drop-out or end
of study (September 1974): 142 prednisone patients and 150 placebo patients
died. The survival times for the remaining patients are
right-censored.
The data file includes the following variables:
ID = patient id. (integer, range 1-561)
DC = 1, if failure 0, if censoring
DAYS = survival time in days from randomization
TMENT = 0, if prednisone 1, if placebo
SEX = 0, if female, 1, if male
ASC = 0, if no ascites, 1, if some, 2, if moderate or marked
AGE (in years, range 17-80)
PRO(thrombin) (in % of normal, range 12-135)
ACE(tylcholinesterase) (in micromoles/min/ml, range 26-659)
INFL(ammation in liver connective tissue) = 0, if none, 1, if slight, 2, if
moderate, 3, if severe
The dataset
Scripts for loading the dataset
Programs related to CSL
| Table 1.5.2: R-source SAS-source (tab-csl-ch1ascbeh) | 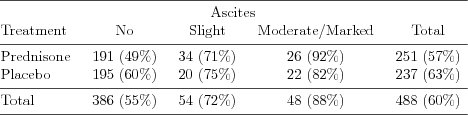 |
| Table 5.2.7: R-source SAS-source (tab-csl-ch5ascbeh-nointeract) | 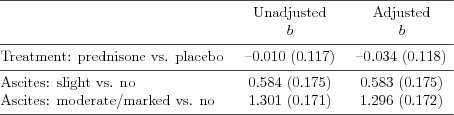 |
| Table 5.2.8: R-source SAS-source (tab-csl-ch5ascbeh-interact1) | 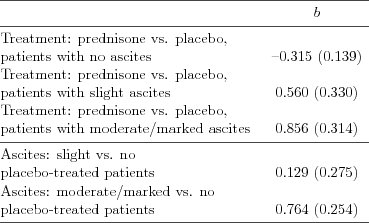 |
| Table 5.2.9: R-source SAS-source (tab-csl-ch5ascbeh-interact2) | 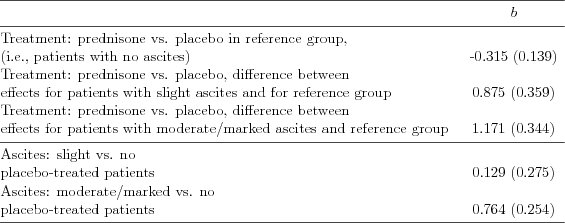 |
| Table 5.2.10: R-source SAS-source (tab-csl-ch5ascbeh-tests) | 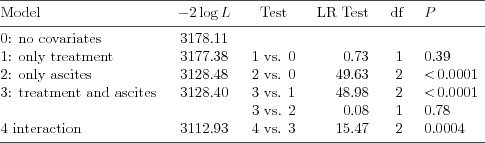 |
| Table 5.2.14: R-source SAS-source (tab-csl-ch5ascbeh-interact3) | 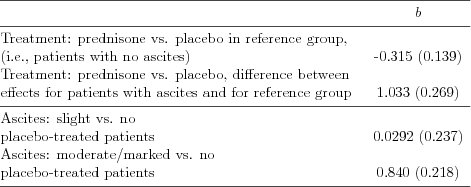 |
| Table 5.3.2: R-source SAS-source (tab-ch5-3wayinter-est) | 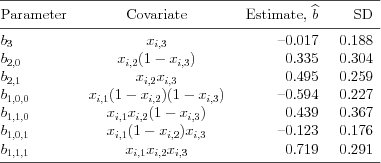 |
| Table 5.3.3: R-source SAS-source (tab-ch5-treatmenteffects) | 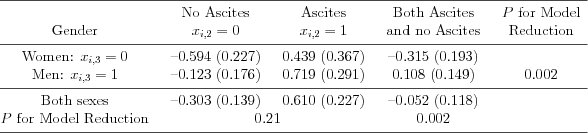 |
